Details of the Drug
General Information of Drug (ID: DMT9FH3)
| Drug Name |
Terlipressin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Glypressin; Lucassin; Terlipressina; Terlipressine; Terlipressinum; Glypressin (TN); HS-2028; Terlipressin [BAN:INN]; Terlipressina [INN-Spanish]; Terlipressine [INN-French]; Terlipressinum [INN-Latin]; N-(N-(N-Glycylglycyl)glycyl)-8-L-lysinevasopressin
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Vasoconstrictor Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
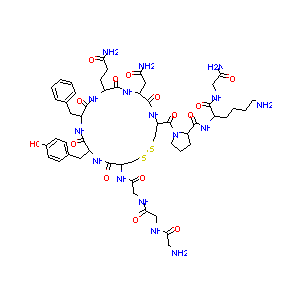 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 1227.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -5.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 25 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 16 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 19 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hypertension | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BA00-BA04 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
